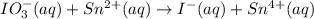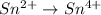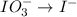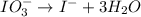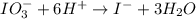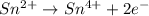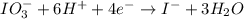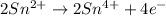Given the partial equation:
IO³⁻ (aq) + Sn²⁺ (aq) → I⁻ (aq) + Sn⁴⁺ (aq),
balance the reaction in acidic solution using the half-reaction method and fill in the coefficients.
The missing blanks represent H2O, H+, or OH-, as required to balance the reaction. Enter the coefficients as integers, using the lowest whole numbers. If the coefficient for something is "1", make sure to type that in and not leave it blank. Enter only the coefficients.
IO³⁻ (aq) + Sn²⁺ (aq) + → I⁻ (aq) + Sn⁴⁺ (aq) +

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2


Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1
You know the right answer?
Given the partial equation:
IO³⁻ (aq) + Sn²⁺ (aq) → I⁻ (aq) + Sn⁴⁺ (aq),
balance the r...
IO³⁻ (aq) + Sn²⁺ (aq) → I⁻ (aq) + Sn⁴⁺ (aq),
balance the r...
Questions

Mathematics, 03.08.2019 08:00

Mathematics, 03.08.2019 08:00

Mathematics, 03.08.2019 08:00

Health, 03.08.2019 08:00



Mathematics, 03.08.2019 08:00


Computers and Technology, 03.08.2019 08:00


Mathematics, 03.08.2019 08:00

Geography, 03.08.2019 08:00

Mathematics, 03.08.2019 08:00

Social Studies, 03.08.2019 08:00

Health, 03.08.2019 08:00

Chemistry, 03.08.2019 08:00



Computers and Technology, 03.08.2019 08:00

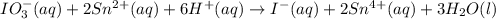
 at that side where the less number of hydrogen are present.
at that side where the less number of hydrogen are present.