
Chemistry, 15.04.2020 00:18 sarinaneedshelp01
A chemist prepares a solution by adding 258 mg of K2Cr2O7 (MW = 294.19 g/mol ) to a volumetric flask, and then adding water until the total volume of the contents of the flask reaches the calibration line that indicates 500 mLmL. Determine the molarity of the prepared solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3


Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
A chemist prepares a solution by adding 258 mg of K2Cr2O7 (MW = 294.19 g/mol ) to a volumetric flask...
Questions


Mathematics, 08.04.2021 18:00

English, 08.04.2021 18:00

History, 08.04.2021 18:00



History, 08.04.2021 18:00




History, 08.04.2021 18:00

Health, 08.04.2021 18:00

Computers and Technology, 08.04.2021 18:00




Mathematics, 08.04.2021 18:00


English, 08.04.2021 18:00

Mathematics, 08.04.2021 18:00



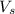 = volume of solution in ml = 500 ml
= volume of solution in ml = 500 ml



