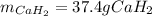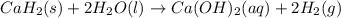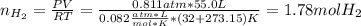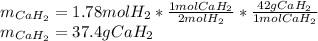Chemistry, 15.04.2020 00:28 swaggirllely36
Calcium hydride (CaH2) reacts with water to form hydrogen gas: CaH2(s) + 2 H2O(l) → Ca(OH)2(aq) + 2 H2(g) Determine the number of grams of CaH2 are needed to generate 55.0 L of H2 gas at a pressure of 0.811 atm and a temperature of 32°C.\

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1



You know the right answer?
Calcium hydride (CaH2) reacts with water to form hydrogen gas: CaH2(s) + 2 H2O(l) → Ca(OH)2(aq) + 2...
Questions


Mathematics, 31.03.2020 15:38

English, 31.03.2020 15:38

English, 31.03.2020 15:38




English, 31.03.2020 15:38

Mathematics, 31.03.2020 15:39


English, 31.03.2020 15:39


Mathematics, 31.03.2020 15:39


Mathematics, 31.03.2020 15:39


Physics, 31.03.2020 15:39

Mathematics, 31.03.2020 15:39

Biology, 31.03.2020 15:39