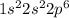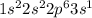Chemistry, 15.04.2020 00:24 twinkle713derp
7.47 Two atoms have the electron configurations 1s22s22p6 and 1s22s22p63s1. The first ionization energy of one is 2080 kJ/mol and that of the other is 496 kJ/mol. Match each ionization energy with one of the given electron configurations.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1


Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
7.47 Two atoms have the electron configurations 1s22s22p6 and 1s22s22p63s1. The first ionization ene...
Questions

Social Studies, 26.02.2021 18:50


Mathematics, 26.02.2021 18:50

Mathematics, 26.02.2021 18:50


Business, 26.02.2021 18:50

English, 26.02.2021 18:50


Mathematics, 26.02.2021 18:50




Mathematics, 26.02.2021 18:50


Mathematics, 26.02.2021 18:50

Mathematics, 26.02.2021 18:50


Social Studies, 26.02.2021 18:50

Biology, 26.02.2021 18:50

History, 26.02.2021 18:50