Chemistry, 15.04.2020 00:52 calebcoolbeans6691
A chemist titrates 160.0 mL of a 0.3065 M cyanic acid (HCNO) solution with 0.4994 M NaOH solution at 25 °C. Calculate the pH at equivalence. The pK of cyanic acid is 3.46 Round your answer to 2 decimal places.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
A chemist titrates 160.0 mL of a 0.3065 M cyanic acid (HCNO) solution with 0.4994 M NaOH solution at...
Questions

Chemistry, 20.11.2019 03:31





Mathematics, 20.11.2019 03:31






English, 20.11.2019 03:31





Chemistry, 20.11.2019 03:31

Chemistry, 20.11.2019 03:31

Mathematics, 20.11.2019 03:31

Mathematics, 20.11.2019 03:31

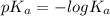
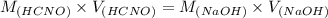
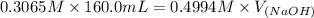
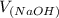 = 98.1978 mL
= 98.1978 mL
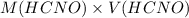
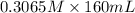
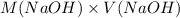
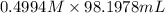
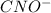 and
and 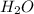 .
.
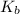 of
of 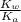
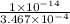

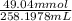
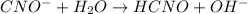
![K_{b} = \frac{[HCNO][OH^{-}]}{[CNO^{-}]}](/tpl/images/0600/3810/7fa50.png)
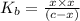
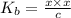
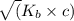
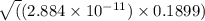
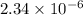
![[OH^{-}] = x = 2.34 \times 10^{-6}](/tpl/images/0600/3810/bb369.png) M
M
![-log [OH^{-}]](/tpl/images/0600/3810/337a4.png)