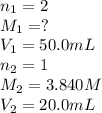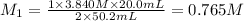Chemistry, 15.04.2020 01:00 dewillis9380
Carbonic acid, H2CO3, has two acidic hydrogens. A solution containing an unknown concentration of carbonic acid is titrated with potassium hydroxide. It requires 20.0 mL of 3.840 M KOH solution to titrate both acidic protons in 50.2 mL of the carbonic acid solution.
1. Write a balanced net ionic equation for the neutralization reaction. Include physical states.
2. Calculate the molarity of the carbonic acid solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Carbonic acid, H2CO3, has two acidic hydrogens. A solution containing an unknown concentration of ca...
Questions

Arts, 06.11.2020 01:00


Mathematics, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

English, 06.11.2020 01:00

Biology, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

Biology, 06.11.2020 01:00

English, 06.11.2020 01:00



English, 06.11.2020 01:00


Health, 06.11.2020 01:00


Physics, 06.11.2020 01:00




History, 06.11.2020 01:00

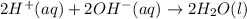
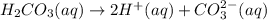 ..[1]
..[1]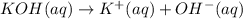 ..[2]
..[2]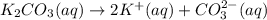 ..[3]
..[3]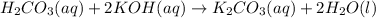
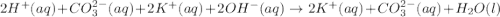
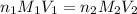
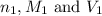 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 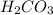
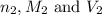 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.