
Chemistry, 15.04.2020 01:19 taytaycola223
Bohr Model: an electron in a doubly ionized lithium atom +2Li(three protons in the nucleus) makes a transition from the 풏=ퟏto the 풏=ퟑlevel with an associated photon. a)Determine the photon energy associated with this transition. (10pts)

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
Bohr Model: an electron in a doubly ionized lithium atom +2Li(three protons in the nucleus) makes a...
Questions




Chemistry, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50

Health, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50


Mathematics, 07.12.2020 19:50



Mathematics, 07.12.2020 19:50

Social Studies, 07.12.2020 19:50

English, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50

History, 07.12.2020 19:50


Chemistry, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50

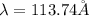
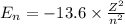
![\Delta E=-13.6\times Z^2[\frac{1}{n_3^2} -\frac{1}{n_1^2}]](/tpl/images/0600/5165/72afd.png)
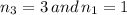
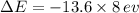
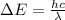
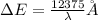
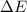 we get,
we get,

