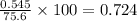Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Consider the reaction CaCN2 + 3 H2O → CaCO3 + 2 NH3 . This reaction has a 75.6% yield. How many mole...
Questions








Mathematics, 23.04.2021 06:10


Mathematics, 23.04.2021 06:10

Mathematics, 23.04.2021 06:10

Mathematics, 23.04.2021 06:10

Mathematics, 23.04.2021 06:10


Mathematics, 23.04.2021 06:10

Mathematics, 23.04.2021 06:10

English, 23.04.2021 06:10

Chemistry, 23.04.2021 06:10

English, 23.04.2021 06:10

Biology, 23.04.2021 06:10

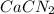 are needed to obtain 18.6 g of
are needed to obtain 18.6 g of 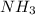
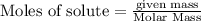
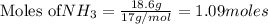
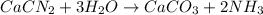
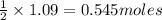 of
of