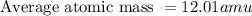Chemistry, 15.04.2020 01:49 RiceLord9562
Jmap Base your answer to the question on the information. Naturally occurring elemental carbon is a mixture of isotopes. The percent composition of the two most abundant isotopes is listed below.98.93% of the carbon atoms have a mass of 12.00 atomic mass units.1.07% of the carbon atoms have a mass of 13.00 atomic mass units. Calculate the average atomic mass of carbon.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Jmap Base your answer to the question on the information. Naturally occurring elemental carbon is a...
Questions


Chemistry, 01.10.2019 23:10



Mathematics, 01.10.2019 23:10

Mathematics, 01.10.2019 23:10








Mathematics, 01.10.2019 23:10


History, 01.10.2019 23:10

Physics, 01.10.2019 23:10


Mathematics, 01.10.2019 23:10

Mathematics, 01.10.2019 23:10

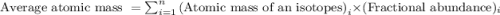
![\text{Average atomic mass }=[(12.00\times 0.9893)+(13\times 0.0107)]](/tpl/images/0600/6598/dc2f3.png)