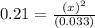Chemistry, 15.04.2020 02:43 prayingfeet7799
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium concentration of NO2(g). N2O4(g) ↔ 2 NO2(g) Kc = 0.21 [N2O4]eq = 0.033 M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1


Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

You know the right answer?
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particula...
Questions

Mathematics, 15.07.2020 19:01








Engineering, 15.07.2020 19:01








Chemistry, 15.07.2020 19:01

Mathematics, 15.07.2020 19:01

Mathematics, 15.07.2020 19:01

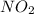 is 0.083 M
is 0.083 M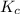
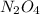 at equilibrium = 0.033 M
at equilibrium = 0.033 M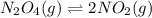
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0600/8631/271f5.png)