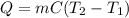In a coffee-cup calorimeter, 100.0 g of H2O and 100.0 mL of HCl are mixed. The HCl had an initial temperature of 44.6 oC and the water was originally at 24.6 oC. After the reaction, the temperature of both substances is 31.3 oC.
a. Was the reaction exothermic or endothermic?Explain.
b. Calculate how much heat the water lost or gained.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
In a coffee-cup calorimeter, 100.0 g of H2O and 100.0 mL of HCl are mixed. The HCl had an initial te...
Questions





Advanced Placement (AP), 13.03.2021 02:30

Mathematics, 13.03.2021 02:30

Chemistry, 13.03.2021 02:30



Mathematics, 13.03.2021 02:30


Mathematics, 13.03.2021 02:30

Chemistry, 13.03.2021 02:30



Mathematics, 13.03.2021 02:30




Biology, 13.03.2021 02:30