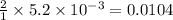A chemist prepares a solution of magnesium chloride MgCl2 by measuring out 49.mg of MgCl2 into a 100.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl−anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3


Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
A chemist prepares a solution of magnesium chloride MgCl2 by measuring out 49.mg of MgCl2 into a 100...
Questions



Mathematics, 21.04.2021 19:20



Mathematics, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20

History, 21.04.2021 19:20

Social Studies, 21.04.2021 19:20



Physics, 21.04.2021 19:20

English, 21.04.2021 19:20

Arts, 21.04.2021 19:20



Mathematics, 21.04.2021 19:20

Biology, 21.04.2021 19:20

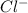 anions in the chemist's solution is 0.0104 M
anions in the chemist's solution is 0.0104 M
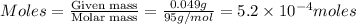
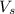 = volume of solution in ml = 100 ml
= volume of solution in ml = 100 ml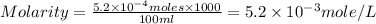
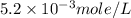
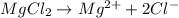
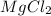 gives 2 moles of
gives 2 moles of 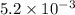 moles of
moles of