Chemistry, 15.04.2020 03:41 sammysosa121832
The energy needed to ionize an atom of si when it is in the most stable is 786.4 kJ mol^-1 however if an atom of Si is in certain low lying excited state only 310.8 is needed to ionize.
what is the wavelength of he radiation emitted when an atom of si undergoes a transition from this excited state to the ground state?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
The energy needed to ionize an atom of si when it is in the most stable is 786.4 kJ mol^-1 however i...
Questions


Geography, 10.04.2020 20:29






Chemistry, 10.04.2020 20:29









Mathematics, 10.04.2020 20:29



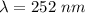
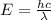
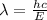 ----- (1)
----- (1)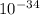 J s
J s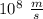
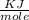
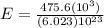
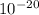 J
J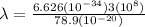
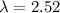 ×
×