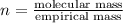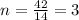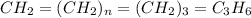Chemistry, 15.04.2020 02:55 bvbbridesmaid5519
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At standard conditions, 112 mL of the gaseous compound weighs 0.21 g. What is the molecular formula for the compound

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1
You know the right answer?
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At st...
Questions

Mathematics, 01.12.2021 22:00

History, 01.12.2021 22:00



Social Studies, 01.12.2021 22:00

Chemistry, 01.12.2021 22:00

Mathematics, 01.12.2021 22:00





Mathematics, 01.12.2021 22:00


Physics, 01.12.2021 22:00

Mathematics, 01.12.2021 22:00

History, 01.12.2021 22:00

Mathematics, 01.12.2021 22:00

Biology, 01.12.2021 22:00


Mathematics, 01.12.2021 22:00

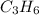
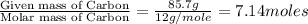
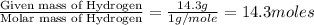
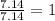
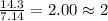
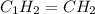
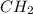 = 1(12) + 2(1) = 14 g/eq.
= 1(12) + 2(1) = 14 g/eq.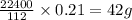 of compound
of compound