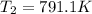Chemistry, 15.04.2020 02:57 christiannpettyy
The compound 1,1-difluoroethane decomposes at elevated temperatures to give fluoroethylene and hydrogen fluoride: CH3CHF2(g) → CH2CHF(g) + HF(g) At 460 °C, k = 5.8 × 10-6 s-1 and Ea = 265 kJ/mol. To what temperature (in K) would you have to raise the reaction to make it go four times as fast?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
The compound 1,1-difluoroethane decomposes at elevated temperatures to give fluoroethylene and hydro...
Questions




Chemistry, 04.07.2019 07:30



Mathematics, 04.07.2019 07:30

Mathematics, 04.07.2019 07:30



Mathematics, 04.07.2019 07:30



Spanish, 04.07.2019 07:30

English, 04.07.2019 07:30



Spanish, 04.07.2019 07:30


Spanish, 04.07.2019 07:30

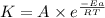
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0600/9306/6d953.png)
 = rate constant at
= rate constant at  =
= 
 = rate constant at
= rate constant at 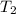 =
= 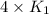
 = activation energy for the reaction = 265 kJ/mol = 265000 J/mol
= activation energy for the reaction = 265 kJ/mol = 265000 J/mol = initial temperature =
= initial temperature = 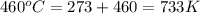
![\log (\frac{4\times K_1}{K_1})=\frac{265000J/mol}{2.303\times 8.314J/mole.K}[\frac{1}{733K}-\frac{1}{T_2}]](/tpl/images/0600/9306/ddf14.png)