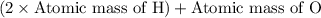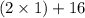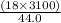Chemistry, 15.04.2020 03:07 nforrestall
A man ate 0.489 pound of cheese (an energy intake of 3.10 × 103 kJ). Suppose that none of the energy was stored in his body. What mass (in grams) of water would he need to perspire in order to maintain his original temperature? (It takes 44.0 kJ to vaporize 1 mole of water.) Enter your answer in scientific notation.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which one of the following gases is not an important component of soil?
Answers: 2

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
A man ate 0.489 pound of cheese (an energy intake of 3.10 × 103 kJ). Suppose that none of the energy...
Questions

Biology, 02.03.2021 21:50


Mathematics, 02.03.2021 21:50

Health, 02.03.2021 21:50


English, 02.03.2021 21:50

History, 02.03.2021 21:50

Biology, 02.03.2021 21:50

Social Studies, 02.03.2021 21:50


History, 02.03.2021 21:50


Social Studies, 02.03.2021 21:50



English, 02.03.2021 21:50



Mathematics, 02.03.2021 21:50