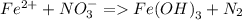Consider the following unbalanced redox reactions. In each case, separate the whole reactions into half-reactions, balance the half reactions, and combine to yield a balanced whole reaction. Compute the net reduction potential (E°) and pε° values for the net reactions and indicate whether the reaction is spontaneous as written. The reduction potential for Fe(OH)_3(s)/Fe^2+ is -0.181 V and pε is -3.08.
a. Fe^2 + + NO_3 = Fe(OH)_3(s) + N_2
b. Mn^2 + + Fe(OH)_3(s) = MnO_2(s) + Fe^2+

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1
You know the right answer?
Consider the following unbalanced redox reactions. In each case, separate the whole reactions into h...
Questions

History, 18.12.2020 21:20

English, 18.12.2020 21:20

Mathematics, 18.12.2020 21:20

Social Studies, 18.12.2020 21:20


Chemistry, 18.12.2020 21:20

History, 18.12.2020 21:20

Biology, 18.12.2020 21:20

Mathematics, 18.12.2020 21:20

Mathematics, 18.12.2020 21:20



History, 18.12.2020 21:20



Arts, 18.12.2020 21:20


Chemistry, 18.12.2020 21:20