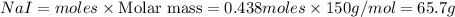Chemistry, 15.04.2020 03:34 alekvtaylor
Iodine is prepared both in the laboratory and commercially by adding Cl2(g) to an aqueous solution containing sodium iodide: 2NaI(aq) +Cl2(g) → I2(s) +2NaCl(aq) How many grams of iodide, NaI, must be used to produce 55.6 g of iodine, I2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
Iodine is prepared both in the laboratory and commercially by adding Cl2(g) to an aqueous solution c...
Questions


Chemistry, 01.02.2022 22:40

Mathematics, 01.02.2022 22:40


History, 01.02.2022 22:40

History, 01.02.2022 22:40


Mathematics, 01.02.2022 22:40


Mathematics, 01.02.2022 22:40






Mathematics, 01.02.2022 22:40




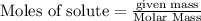
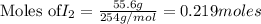
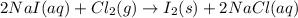
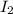 are produced by = 2 moles of
are produced by = 2 moles of 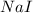
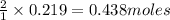 of
of