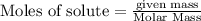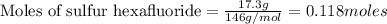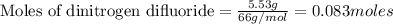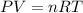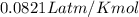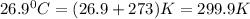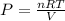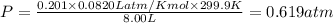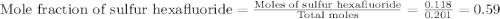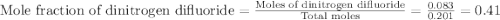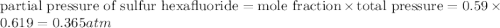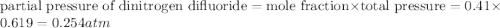Chemistry, 15.04.2020 03:30 briarwilliams9668
A tank at is filled with of chlorine pentafluoride gas and of dinitrogen difluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Be sure your answers have the correct number of significant digits. chlorine pentafluoride mole fraction: partial pressure: dinitrogen difluoride mole fraction: partial pressure: Total pressure in tank:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

You know the right answer?
A tank at is filled with of chlorine pentafluoride gas and of dinitrogen difluoride gas. You can ass...
Questions

Mathematics, 18.10.2019 01:50


Biology, 18.10.2019 01:50


Mathematics, 18.10.2019 01:50


Mathematics, 18.10.2019 01:50





Chemistry, 18.10.2019 01:50

History, 18.10.2019 01:50



English, 18.10.2019 01:50

Mathematics, 18.10.2019 01:50

Mathematics, 18.10.2019 01:50