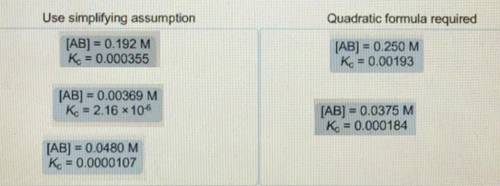
When determining the equilibrium concentrations for the reaction ; a simplifying assumption can be used under certain conditions to avoid solving a quadratic equation. Classify each situation by whether the simplifying assumption can be used or whether the quadratic formula is required. Use simplifying assumptions - Quadratic formula required 1. [AB] = 0.0178 M; 2. [AB] = 0.00204 M; 3. [AB] =0.451 M; = 0.000905 4. [AB] = 0.0174 M; = 0.0000925 5. [AB] = 0.396 M; = 0.00228

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
When determining the equilibrium concentrations for the reaction ; a simplifying assumption can be...
Questions

Computers and Technology, 13.01.2022 07:30

Geography, 13.01.2022 07:30



Biology, 13.01.2022 07:30



Mathematics, 13.01.2022 07:30




Health, 13.01.2022 07:40


English, 13.01.2022 07:40


Computers and Technology, 13.01.2022 07:40

Social Studies, 13.01.2022 07:40


English, 13.01.2022 07:50

Mathematics, 13.01.2022 07:50