Chemistry, 15.04.2020 03:54 Ashley606hernandez
Diborane (B2H6) is a gas at room temperature that forms explosive mixtures with air. It reacts with oxygen according to the following equation (which may or may not be balanced): B2H6 (g) + O2 (g) → B2O3 (s) + H2O (l) How many grams of O2 (molar mass 32.00 g/mol) will react with 14.67 grams of diborane (molar mass 27.67 g/mol). Your answer must be expressed to the correct number of significant figures, and with the correct unit.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1



Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Diborane (B2H6) is a gas at room temperature that forms explosive mixtures with air. It reacts with...
Questions

Chemistry, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00

Advanced Placement (AP), 07.11.2020 01:00



Mathematics, 07.11.2020 01:00




English, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00


Mathematics, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00

English, 07.11.2020 01:00



Mathematics, 07.11.2020 01:00

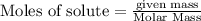
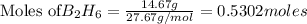
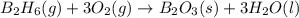
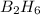 require = 3 moles of
require = 3 moles of 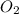
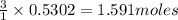 of
of