
Chemistry, 29.08.2019 22:40 austintules2005
How many moles of ethylene (c2h4) can react with 12.9 liters of oxygen gas at 1.2 atmospheres and 297 kelvin?
c2h4(g) + 3o2(g) yields 2co2(g) + 2h2o(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
You know the right answer?
How many moles of ethylene (c2h4) can react with 12.9 liters of oxygen gas at 1.2 atmospheres and 29...
Questions

Social Studies, 21.04.2021 16:40

Business, 21.04.2021 16:40




Social Studies, 21.04.2021 16:40



Chemistry, 21.04.2021 16:40

English, 21.04.2021 16:40




Chemistry, 21.04.2021 16:40

Advanced Placement (AP), 21.04.2021 16:40

Mathematics, 21.04.2021 16:40

Mathematics, 21.04.2021 16:40



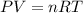
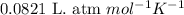

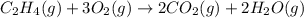
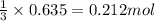 of ethylene gas
of ethylene gas


