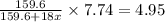Chemistry, 15.04.2020 04:41 Madisonk3571
A hydrated form of copper sulfate ( CuSO 4 ⋅ x H 2 O ) is heated to drive off all of the water. If there is initially 7.74 g of hydrated salt and there is 4.95 g of anhydrous CuSO 4 after heating, find the number of water molecules associated with each CuSO 4 formula unit.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
A hydrated form of copper sulfate ( CuSO 4 ⋅ x H 2 O ) is heated to drive off all of the water. If t...
Questions

Mathematics, 20.03.2020 10:02


Mathematics, 20.03.2020 10:02


Biology, 20.03.2020 10:02






Arts, 20.03.2020 10:02

Arts, 20.03.2020 10:02







Social Studies, 20.03.2020 10:02

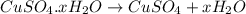
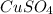 = 159.6 g/mol
= 159.6 g/mol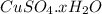 decomposes to give = 159.6g of
decomposes to give = 159.6g of 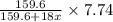 g
g