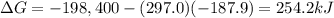For the process 2SO2(g) + O2(g) --> 2SO3(g),
ΔS = –187.9 J/K and ΔH = –198.4 kJ at 29...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 23.06.2019 08:40
A20 liter cylinder of helium at a pressure of 150 atm and a temperature of 27°c is used to fill a balloon at 1.00 atm and 37°c. what is the volume of the balloon? a. 0.14 liters b. 3000 liters c. 2900 liters d. 2400 liters e. 3100 liters
Answers: 1
You know the right answer?
Questions




Health, 05.11.2020 08:00

Spanish, 05.11.2020 08:00

Health, 05.11.2020 08:00

Biology, 05.11.2020 08:00

English, 05.11.2020 08:00



Mathematics, 05.11.2020 08:00


Advanced Placement (AP), 05.11.2020 08:00

History, 05.11.2020 08:00

Biology, 05.11.2020 08:00


Biology, 05.11.2020 08:00


English, 05.11.2020 08:00

English, 05.11.2020 08:00

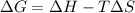
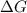 is the Gibbs free energy
is the Gibbs free energy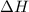 is the change in enthalpy of the reaction
is the change in enthalpy of the reaction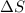 is the change in entropy
is the change in entropy