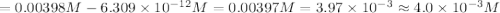Chemistry, 18.10.2019 22:30 cmfuentes0816
The equation for the ph of a substance is ph = –log[h+], where h+ is the concentration of hydrogen ions. a basic solution has a ph of 11.2. an acidic solution has a ph of 2.4. what is the approximate difference in the concentration of hydrogen ions between the two solutions? a) 1.6*10^-9 b) 4.0*10^-3 c)6.7*10^-1 d)1.6*10^-1

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3


Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
The equation for the ph of a substance is ph = –log[h+], where h+ is the concentration of hydrogen i...
Questions



English, 20.09.2019 13:20

Business, 20.09.2019 13:20

Arts, 20.09.2019 13:20


Mathematics, 20.09.2019 13:20

Social Studies, 20.09.2019 13:20

Biology, 20.09.2019 13:20


Mathematics, 20.09.2019 13:20

Mathematics, 20.09.2019 13:20


Mathematics, 20.09.2019 13:20


Social Studies, 20.09.2019 13:20


Mathematics, 20.09.2019 13:20


Chemistry, 20.09.2019 13:20

![pH = -\log[H+]](/tpl/images/0332/6029/30b50.png)
![11.2=-\log[H^+]](/tpl/images/0332/6029/fa6ad.png)
![[H^+]=6.309\times 10^{-12} M](/tpl/images/0332/6029/eb264.png)
![2.4=-\log[H^+]](/tpl/images/0332/6029/156c9.png)
![[H^+]'=0.0039810 M=3.9810\times 10^{-3} M](/tpl/images/0332/6029/c1356.png)
![=[H^+]'-[H^+]](/tpl/images/0332/6029/74fcc.png)