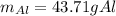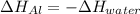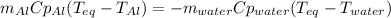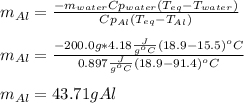Chemistry, 15.04.2020 15:49 ljcervantes4824
A chunk of aluminum at 91.4°C was added to 200.0 g of water at 15.5°C. The specific heat of aluminum is 0.897 J/g°C, and the specific heat of water is 4.18 J/g°C. When the temperature stabilized, the temperature of the mixture was 18.9°C. Assuming no heat was lost to the surroundings, what was the mass of aluminum added?
A. 243 g
B. 34.7 g
C. 41.7 g
D. 43.7 g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
You know the right answer?
A chunk of aluminum at 91.4°C was added to 200.0 g of water at 15.5°C. The specific heat of aluminum...
Questions

History, 08.10.2019 23:30

Mathematics, 08.10.2019 23:30

Mathematics, 08.10.2019 23:30

Mathematics, 08.10.2019 23:30

English, 08.10.2019 23:30



Mathematics, 08.10.2019 23:30

Health, 08.10.2019 23:30


Mathematics, 08.10.2019 23:30


Spanish, 08.10.2019 23:30

Mathematics, 08.10.2019 23:30


Mathematics, 08.10.2019 23:30



Social Studies, 08.10.2019 23:30