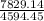Chemistry, 15.04.2020 17:17 kelseatuttleni
A mass of 35.0 g of an unknown solid initially at 170.0 ∘C is added to an ideal constant-pressure calorimeter containing 100.0 g of water (Cs, water=4.184 J/(g⋅∘C)) initially at 20.0 ∘C. After the mixture reaches thermal equilibrium, the final temperature is recorded to be 38.73 ∘C. What is the specific heat capacity of the unknown solid?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2
You know the right answer?
A mass of 35.0 g of an unknown solid initially at 170.0 ∘C is added to an ideal constant-pressure ca...
Questions



English, 29.01.2021 22:00



Mathematics, 29.01.2021 22:00


Arts, 29.01.2021 22:00



Chemistry, 29.01.2021 22:00



Chemistry, 29.01.2021 22:00