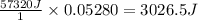A student enters the lab and conducts Part A of the Experiment. The student uses 25.00 mL of 2.112 M HCl, and adds NaOH in excess as instructed. If the ΔH of the neutralization reaction is known to be -57,320 J/mol H2O, what is the total theoretical heat released (in Joules)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1


Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
A student enters the lab and conducts Part A of the Experiment. The student uses 25.00 mL of 2.112 M...
Questions


Mathematics, 17.07.2019 01:30

Mathematics, 17.07.2019 01:30

Biology, 17.07.2019 01:30


English, 17.07.2019 01:30



Social Studies, 17.07.2019 01:30





Mathematics, 17.07.2019 01:30


Mathematics, 17.07.2019 01:30


Biology, 17.07.2019 01:30


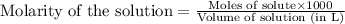 .....(1)
.....(1)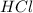 solution = 2.112 M
solution = 2.112 M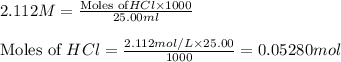

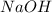 is the excess reagent.
is the excess reagent.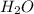
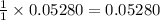 moles of
moles of