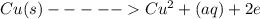Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

You know the right answer?
Given the balanced ionic equation representing a reaction: Cu(s) + 2Ag+(aq) Cu2+(aq) + 2Ag(s) During...
Questions



Mathematics, 13.10.2019 03:30


English, 13.10.2019 03:30

Social Studies, 13.10.2019 03:30

History, 13.10.2019 03:30


Social Studies, 13.10.2019 03:30



Mathematics, 13.10.2019 03:30

English, 13.10.2019 03:30

Mathematics, 13.10.2019 03:30


History, 13.10.2019 03:30


History, 13.10.2019 03:30