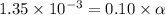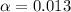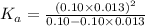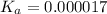Chemistry, 15.04.2020 20:21 tiffanybrandy23
Determine the acid dissociation constant for a 0.10 m acetic acid solution that has a ph of 2.87. Acetic acid is a weak monoprotic acid and the equilibrium equation of interest is

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Determine the acid dissociation constant for a 0.10 m acetic acid solution that has a ph of 2.87. Ac...
Questions




English, 25.04.2020 04:49


Mathematics, 25.04.2020 04:49


Geography, 25.04.2020 04:49

Mathematics, 25.04.2020 04:49

Advanced Placement (AP), 25.04.2020 04:49



Computers and Technology, 25.04.2020 04:49




Mathematics, 25.04.2020 04:49


Mathematics, 25.04.2020 04:50

Mathematics, 25.04.2020 04:50

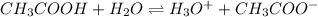
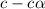
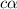
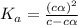
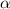 = dissociation constant = ?
= dissociation constant = ?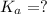
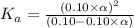
![pH=-log[H^+]](/tpl/images/0602/6192/15713.png)
![2.87=-log[H^+]](/tpl/images/0602/6192/3a07c.png)
![[H^+]=1.35\times 10^{-3}](/tpl/images/0602/6192/8f9f0.png)
![[H^+]=c\times \alpha](/tpl/images/0602/6192/4fc41.png)