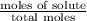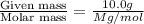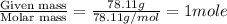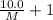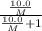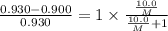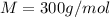Chemistry, 15.04.2020 19:55 gandalfhan
At a certain temperature, the vapor pressure of pure benzene (C6H6) is 0.930 atm. A solution was prepared by dissolving 10.0 g of a nondissociating, nonvolatile solute in 78.11g of benzene at that temperature. The vapor pressure of the solution was found to be 0.900 atm. Assuming the solution behaves ideally, determine the molar mass of the solute.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

You know the right answer?
At a certain temperature, the vapor pressure of pure benzene (C6H6) is 0.930 atm. A solution was pre...
Questions

History, 13.07.2019 10:00

Mathematics, 13.07.2019 10:00

Business, 13.07.2019 10:00


Biology, 13.07.2019 10:00

Physics, 13.07.2019 10:00

History, 13.07.2019 10:00


History, 13.07.2019 10:00

Mathematics, 13.07.2019 10:00

Biology, 13.07.2019 10:00


Social Studies, 13.07.2019 10:00



Social Studies, 13.07.2019 10:00

Social Studies, 13.07.2019 10:00



Chemistry, 13.07.2019 10:00

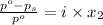
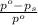 = relative lowering in vapor pressure
= relative lowering in vapor pressure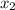 = mole fraction of solute =
= mole fraction of solute =