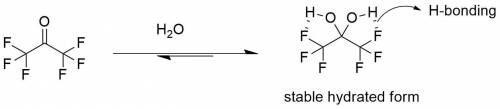
For most ketones, hydrate formation is unfavorable, because the equilibrium favors the ketone rather than the hydrate. However, the equilibrium for hydration of hexafluoroacetone favors formation of the hydrate. Provide a plausible explanation for this observation.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
For most ketones, hydrate formation is unfavorable, because the equilibrium favors the ketone rather...
Questions

Chemistry, 29.09.2020 23:01

SAT, 29.09.2020 23:01





Chemistry, 29.09.2020 23:01




Computers and Technology, 29.09.2020 23:01





Medicine, 29.09.2020 23:01

Mathematics, 29.09.2020 23:01



Biology, 29.09.2020 23:01

 can not escape the hydrated form and goes back to keto form.
can not escape the hydrated form and goes back to keto form.