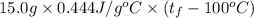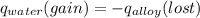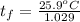Chemistry, 15.04.2020 20:48 jforeman42
A 15.0 g sample of nickel metal is heated to 100.0 degrees C and dropped into 55.0 g of water, initially at 23.0 degrees C. Assuming that all the heat lost by nickel is absorbed by the water, calculate the final temperature of the nickel and water. (C

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Which is the most likely way an automotive engineer would use chemistry
Answers: 1


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
A 15.0 g sample of nickel metal is heated to 100.0 degrees C and dropped into 55.0 g of water, initi...
Questions


Mathematics, 12.02.2021 03:50

English, 12.02.2021 03:50


Mathematics, 12.02.2021 03:50

Mathematics, 12.02.2021 03:50

Mathematics, 12.02.2021 03:50


Mathematics, 12.02.2021 03:50



Mathematics, 12.02.2021 03:50






Advanced Placement (AP), 12.02.2021 03:50

Mathematics, 12.02.2021 03:50

 .
.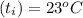 ,
, 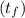 = ?,
= ?,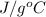 ,
, 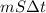
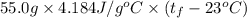
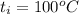 ,
,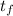 = ?
= ?