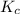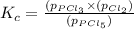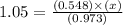Chemistry, 15.04.2020 21:03 soleydyperez
The equilibrium constant (Kp) for the decomposition of phosphorus pentachloride (PCl5) to phosphorus trichloride (PCl3) and molecular chlorine (Cl2) is found to be 1.05 at 250oC. If the equilibrium partial pressure of PCl5 and PCl3 are 0.973 and 0.548 atm, respectively, what is the equilibrium partial pressure of Cl2 at 250 oC?PCl5 (g) ↔ PCl3 (g) + Cl2 (g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

You know the right answer?
The equilibrium constant (Kp) for the decomposition of phosphorus pentachloride (PCl5) to phosphorus...
Questions




English, 04.03.2020 23:18




Mathematics, 04.03.2020 23:19









Mathematics, 04.03.2020 23:19



 is 1.86 atm
is 1.86 atm