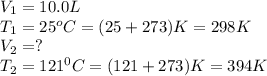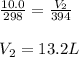Chemistry, 15.04.2020 20:57 blakesmith0110
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 629 torr. Using Charles's law to calculate the volume (L) the gas will occupy when the temperature is increased 121 °C while maintaining the pressure at 629 torr.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

You know the right answer?
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 629 torr. Using Ch...
Questions



Mathematics, 24.05.2021 20:00




Mathematics, 24.05.2021 20:00

Mathematics, 24.05.2021 20:00

Mathematics, 24.05.2021 20:00


Mathematics, 24.05.2021 20:00


Mathematics, 24.05.2021 20:00


Mathematics, 24.05.2021 20:00

Business, 24.05.2021 20:00

Mathematics, 24.05.2021 20:00

History, 24.05.2021 20:00

English, 24.05.2021 20:00

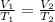
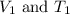 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.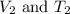 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.