Chemistry, 15.04.2020 21:59 chloejason8375
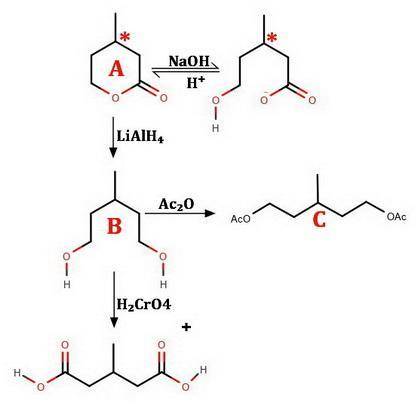
An optically active compound A, C6H10O2, when dissolved in NaOH solution, consumed one equivalent of base. On acidification, compound A was slowly regenerated. Treatment of A with LiAlH4 in ether followed by protonolysis gave an optically inactive compound B that reacted with acetic anhydride to give an acetate diester derivative C. Compound B was oxidized by aqueous chromic acid to β-methylglutaric acid (3-methylpentanedioic acid), D. Identify compounds A, B, and C; do not specify stereochemistry. (The absolute stereochemical configurations of chiral substances cannot be determined from the data.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2


Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

You know the right answer?
An optically active compound A, C6H10O2, when dissolved in NaOH solution, consumed one equivalent of...
Questions

Mathematics, 07.05.2021 21:40





Mathematics, 07.05.2021 21:40

English, 07.05.2021 21:40

Mathematics, 07.05.2021 21:40




Mathematics, 07.05.2021 21:40


Health, 07.05.2021 21:40


Mathematics, 07.05.2021 21:40


Mathematics, 07.05.2021 21:40

Mathematics, 07.05.2021 21:40