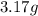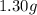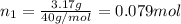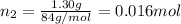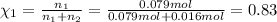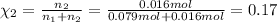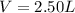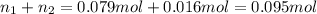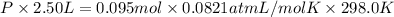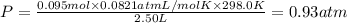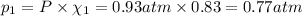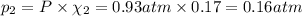Chemistry, 15.04.2020 22:05 cjjjjjjjjjjjjj
If a gaseous mixture is made by combining 3.17 g Ar 3.17 g Ar and 1.30 g Kr 1.30 g Kr in an evacuated 2.50 L container at 25.0 ∘ C, 25.0 ∘C, what are the partial pressures of each gas, P Ar PAr and P Kr , PKr, and what is the total pressure, P total , Ptotal, exerted by the gaseous mixture?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2


Chemistry, 23.06.2019 08:30
Sand is more likely than shale to preserve fossils. true false
Answers: 2
You know the right answer?
If a gaseous mixture is made by combining 3.17 g Ar 3.17 g Ar and 1.30 g Kr 1.30 g Kr in an evacuate...
Questions

English, 11.05.2021 01:00


Mathematics, 11.05.2021 01:00

Chemistry, 11.05.2021 01:00


English, 11.05.2021 01:00






Mathematics, 11.05.2021 01:00

English, 11.05.2021 01:00

English, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00


Mathematics, 11.05.2021 01:00


Mathematics, 11.05.2021 01:00