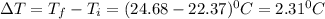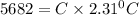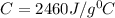Two experiments were conducted in a bomb calorimeter. The first one to determine the heat capacity of the calorimeter, the second the heat of combustion of the carcinogenic substance benzene (C6H6). a. In the first experiment, the temperature rises from 22.37 o C to 24.68 o C when the calorimeter absorbs 5682 J of heat. Determine the heat capacity of the calorimeter.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1



Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
Two experiments were conducted in a bomb calorimeter. The first one to determine the heat capacity o...
Questions

History, 27.04.2021 06:30



History, 27.04.2021 06:30


Social Studies, 27.04.2021 06:30

Mathematics, 27.04.2021 06:30


Mathematics, 27.04.2021 06:30

Biology, 27.04.2021 06:30

Mathematics, 27.04.2021 06:30

Mathematics, 27.04.2021 06:30

Social Studies, 27.04.2021 06:30

Chemistry, 27.04.2021 06:30

Mathematics, 27.04.2021 06:30


Chemistry, 27.04.2021 06:30

Mathematics, 27.04.2021 06:30


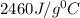
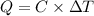
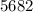 Joules
Joules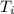 = 22.37°C
= 22.37°C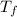 = 24.68°C
= 24.68°C