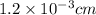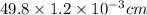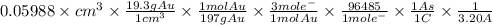Chemistry, 15.04.2020 23:16 lilsnsbsbs
The surface area of an object to be gold plated is 49.8 cm2 and the density of gold is 19.3 g/cm-3. A current of 3.15. A is applied to a solution that contains gold in the +3 oxidation state. Calculate the time required to deposit an even layer of gold 1.2 x 10 -3cm thick on the object.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1


Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
The surface area of an object to be gold plated is 49.8 cm2 and the density of gold is 19.3 g/cm-3....
Questions

Chemistry, 10.12.2021 02:00

Mathematics, 10.12.2021 02:00

Chemistry, 10.12.2021 02:00

History, 10.12.2021 02:00

Mathematics, 10.12.2021 02:00


English, 10.12.2021 02:00



Physics, 10.12.2021 02:00


Biology, 10.12.2021 02:00

Biology, 10.12.2021 02:00

Mathematics, 10.12.2021 02:00


French, 10.12.2021 02:00




 sec.
sec.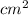 ,
,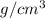 ,
,