

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1
You know the right answer?
AB2 is a molecule that reacts readily with water. Calculate the bond energy of the A–B bond using th...
Questions

History, 19.04.2020 20:43


Mathematics, 19.04.2020 20:43



History, 19.04.2020 20:44


History, 19.04.2020 20:44

Mathematics, 19.04.2020 20:44





Mathematics, 19.04.2020 20:44

Mathematics, 19.04.2020 20:44

Mathematics, 19.04.2020 20:45

Mathematics, 19.04.2020 20:45



Biology, 19.04.2020 20:45

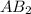 is a molecule that reacts readily with water. Calculate the bond energy of the A–B bond using the standard enthalpy of reaction and the bond energy data provided. Enter a number in kJ to 0 decimal places.
is a molecule that reacts readily with water. Calculate the bond energy of the A–B bond using the standard enthalpy of reaction and the bond energy data provided. Enter a number in kJ to 0 decimal places. 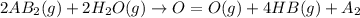
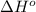 = –142 kJ
= –142 kJ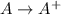
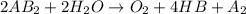
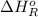 = -142 kJ
= -142 kJ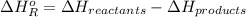
![[(2 \times 2 (A-B) + 2 \times 2 (O-H)] - [(O=O) + 4(H-B) + (A-A)]](/tpl/images/0603/6629/cfc5d.png)
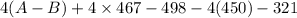
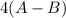 = -142 - 1868 + 498 + 1800 + 321
= -142 - 1868 + 498 + 1800 + 321

