

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

You know the right answer?
An ideal gas originally at 0.85 atm and 66°C was allowed to expand until its final volume, pressure...
Questions


History, 21.07.2019 12:30

Social Studies, 21.07.2019 12:30


Chemistry, 21.07.2019 12:30





English, 21.07.2019 12:30



Business, 21.07.2019 12:30


English, 21.07.2019 12:30


Mathematics, 21.07.2019 12:30

Mathematics, 21.07.2019 12:30


Computers and Technology, 21.07.2019 12:30

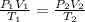
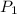 = initial pressure of gas = 0.85 atm
= initial pressure of gas = 0.85 atm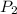 = final pressure of gas = 456 mm Hg = 0.60 atm (760mmHg=1atm)
= final pressure of gas = 456 mm Hg = 0.60 atm (760mmHg=1atm)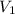 = initial volume of gas = ?
= initial volume of gas = ?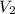 = final volume of gas = 94.0 ml
= final volume of gas = 94.0 ml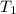 = initial temperature of gas =
= initial temperature of gas = 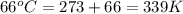
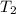 = final temperature of gas =
= final temperature of gas = 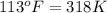
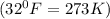
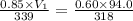
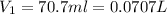 (1L=1000ml)
(1L=1000ml)

