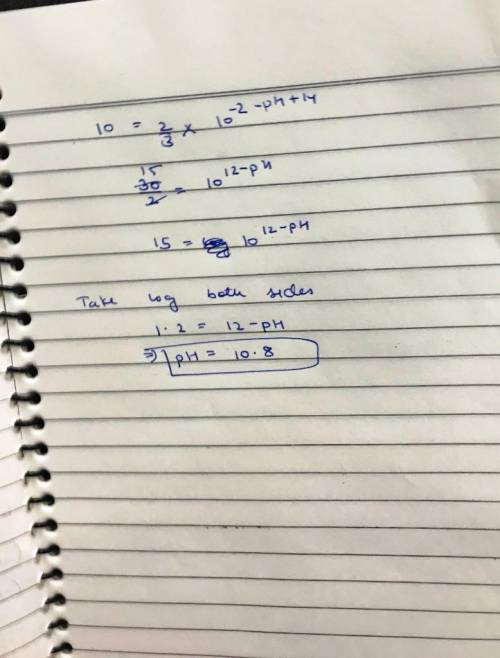Chemistry, 16.04.2020 00:17 sarahhN7534
The decomposition of Bromodichloroacetate BrCl2CCO2- is an important required step in water purification. The kinetics of such decomposition has been presented in Chemical Reviews, November 2001. There are two possible pathways for these reactions, one unimolecular and the other bimolecular with the help of OH- ions.
Path 1. BrCl2CCO2- + H2O goes to CHCl2Br + HCO3-
With a pseudo-first-order rate constant k1=1.6 x 10-6 1/sec
Path 2. BrCl2CCO2- + OH- goes to Cl2OHCCO2- + Br-
With a second-order rate constant k2=2.4x10-4 1/(M sec)
(a) Write the overall rate law for the disappearance of BrCl2CCO2.
(b) What is the ratio of the rates for paths 1 and 2 at pH=7?
(c) At what pH would the rate for path 2 be 10% of the rate for path 1?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

You know the right answer?
The decomposition of Bromodichloroacetate BrCl2CCO2- is an important required step in water purifica...
Questions

Chemistry, 01.09.2021 17:50

Mathematics, 01.09.2021 17:50

Mathematics, 01.09.2021 17:50

Mathematics, 01.09.2021 17:50

English, 01.09.2021 17:50


Mathematics, 01.09.2021 18:00

Mathematics, 01.09.2021 18:00



Mathematics, 01.09.2021 18:00






Mathematics, 01.09.2021 18:00

Mathematics, 01.09.2021 18:00

Mathematics, 01.09.2021 18:00