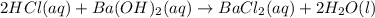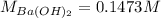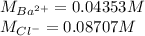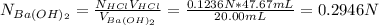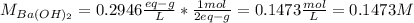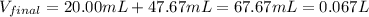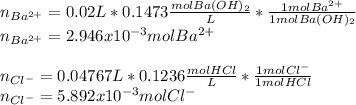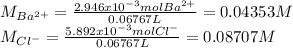A 20.00 mL Ba(OH)_2 solution of unknown concentration was neutralized by the addition of 47.67 mL of a 0.1236 M HCl solution. Write the balanced molecular equation for the neutralization reaction between HCl and Ba(OH)_2 in aqueous solution. Include physical states. 2HCl(aq) + Ba(OH)_2(aq) rightarrow BaCl_2(aq) + 2H_2O(l) Calculate the concentration of Ba(OH)_2 in the original 20.00 mL solution Calculate the concentrations of Ba^2+ and Cl^- in solution following the neutralization reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
A 20.00 mL Ba(OH)_2 solution of unknown concentration was neutralized by the addition of 47.67 mL of...
Questions

French, 06.10.2019 10:01



History, 06.10.2019 10:01



Arts, 06.10.2019 10:01


Chemistry, 06.10.2019 10:01

Physics, 06.10.2019 10:01





English, 06.10.2019 10:01




History, 06.10.2019 10:01