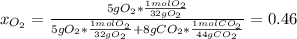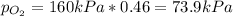Chemistry, 16.04.2020 01:01 genyjoannerubiera
A tank contains an ideal gas mixture of 5 g of O2 and 8 g of CO2 at 160kPa and specified temperature. If O2 were separated from the mixture and stored at mixture temperature and in the same tank, its pressure would be, in kPa (round to nearest integer; for example if the answer is 14.6kPa, write 15; if the answer is 14.49L write 14; do not include the units in your answer

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
A tank contains an ideal gas mixture of 5 g of O2 and 8 g of CO2 at 160kPa and specified temperature...
Questions












Computers and Technology, 15.11.2019 02:31