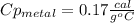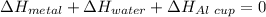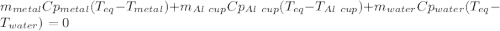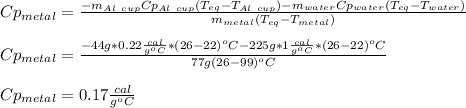Chemistry, 16.04.2020 01:02 isabelperez063
77 grams of an unknown metal at 99ᵒC is placed in 225 grams of water which is initially at 22ᵒC. The water is inside a 44 gram aluminum cup with a specific heat of 0.22 cal/gᵒC. The final temperature of the system is 26ᵒC. What is the specific heat of the unknown metal?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1


Chemistry, 23.06.2019 09:00
Agust of wind blowing east pushes against a ball. when will the wind do work on the ball? when the ball moves to the east when the ball moves to the north when the ball stays in one place when the ball moves north or south
Answers: 1
You know the right answer?
77 grams of an unknown metal at 99ᵒC is placed in 225 grams of water which is initially at 22ᵒC. The...
Questions


Mathematics, 27.05.2021 14:00


Biology, 27.05.2021 14:00

English, 27.05.2021 14:00

Mathematics, 27.05.2021 14:00



Mathematics, 27.05.2021 14:00

Advanced Placement (AP), 27.05.2021 14:00

Business, 27.05.2021 14:00


Mathematics, 27.05.2021 14:00

Health, 27.05.2021 14:00

Social Studies, 27.05.2021 14:00


Business, 27.05.2021 14:00


Biology, 27.05.2021 14:00