
Suppose you add 0.2469 g of PbCl2(s) to 50.0 mL of water. In the resulting saturated solution, you find that the concentration of PbX2+(aq) is 0.0159 M and the concentration of C l − ( a q ) ClX−(aq) is 0.0318 M. What is the value of the equilibrium constant, Ksp, for the dissolution of PbCl2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
Suppose you add 0.2469 g of PbCl2(s) to 50.0 mL of water. In the resulting saturated solution, you f...
Questions

Mathematics, 06.05.2021 22:10







Mathematics, 06.05.2021 22:10



Mathematics, 06.05.2021 22:10





Mathematics, 06.05.2021 22:10

Health, 06.05.2021 22:10

Mathematics, 06.05.2021 22:10

Chemistry, 06.05.2021 22:10

Mathematics, 06.05.2021 22:10

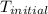 = a - -
= a - -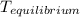 = a - s s 2s
= a - s s 2s

