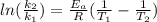The rate constant for a first order reaction is 0.060s-1. when the temperature is increased from 298K to 331 K , the rate constant increases to 0.18s-1. Calculate the activation energy for this reaction. Use kJ/mol for the units. The activation energy in kJ/mol equals (include the units in your answer):

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

You know the right answer?
The rate constant for a first order reaction is 0.060s-1. when the temperature is increased from 298...
Questions

Mathematics, 25.02.2021 01:30


History, 25.02.2021 01:30

Mathematics, 25.02.2021 01:30







Mathematics, 25.02.2021 01:30



Chemistry, 25.02.2021 01:30




Geography, 25.02.2021 01:30


Mathematics, 25.02.2021 01:30