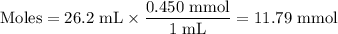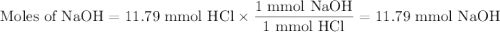Chemistry, 16.04.2020 03:18 Kingmoney959
26.2 mL of a 0.450 M hydrochloric acid solution is titrated with an unknown concentration of sodium hydroxide. 45.8 mL of the sodium hydroxide solution is required to reach the equivalence point. What is the molar concentration of the sodium hydroxide solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2
You know the right answer?
26.2 mL of a 0.450 M hydrochloric acid solution is titrated with an unknown concentration of sodium...
Questions

Mathematics, 23.06.2019 06:20



Mathematics, 23.06.2019 06:20


Computers and Technology, 23.06.2019 06:20

World Languages, 23.06.2019 06:20


History, 23.06.2019 06:20


Chemistry, 23.06.2019 06:20


English, 23.06.2019 06:20


Mathematics, 23.06.2019 06:20