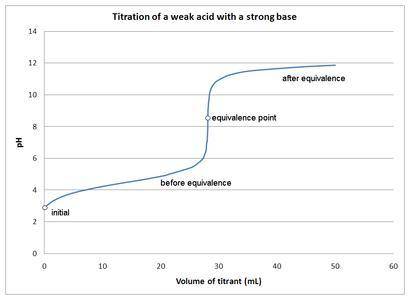
Which of the following is TRUE?
a. The equivalence point is where the amount of acid equals the amount of base during any acid-base titration.
b. At the equivalence point, the pH is always 7.
c. An indicator is not pH sensitive.
d. A titration curve is a plot of pH vs. the [base]/[acid] ratio.
e. None of the above are true.

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 13:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 14:00
Which of the following represents the balanced reduction half-reaction from the redox reaction? (2 points) pb + pd(no3)2 yields pb(no3)2 + pd pb yields pd2+ + e- pd2+ + 2e- yields pd pb + e- yields pb 2 pd2+ + 4 e- yields 2 pd
Answers: 1
You know the right answer?
Which of the following is TRUE?
a. The equivalence point is where the amount of acid equ...
a. The equivalence point is where the amount of acid equ...
Questions



Advanced Placement (AP), 17.01.2020 19:31


Mathematics, 17.01.2020 19:31

Mathematics, 17.01.2020 19:31



Mathematics, 17.01.2020 19:31

Mathematics, 17.01.2020 19:31

Mathematics, 17.01.2020 19:31



Mathematics, 17.01.2020 19:31

Mathematics, 17.01.2020 19:31