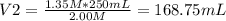You need to prepare 250.0 mL of a 1.35 M HCl solution from a 2.00 M HCl stock solution.
...

Chemistry, 16.04.2020 04:56 Bjehnsen3720
You need to prepare 250.0 mL of a 1.35 M HCl solution from a 2.00 M HCl stock solution.
a. Which glassware should you use to make the solution?
A. beaker
B. Erlenmeyer flask
C. volumetric flask
b. How should the correct amount of stock be obtained?
A. Measure out x g on a balance
B. Measure out x mL using a volumetric pipet
C. Measure out x mL using a graduated cylinder
c. Based on your answer above, what is the value of x?
d. How should the solution be mixed together?
A. Fill the container to the 250 mL mark then add the correct amount of stock solution.
B. Add the correct amount of stock solution then fill to the 250 mL mark with water.
C. Fill the container partially with water, add the correct amount of stock solution, then fill to the 250 mL mark with water.
D. None of these is the correct way to mix the stock solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
Questions

Mathematics, 22.10.2019 15:00

English, 22.10.2019 15:00

Social Studies, 22.10.2019 15:00

Mathematics, 22.10.2019 15:00



Mathematics, 22.10.2019 15:00

Mathematics, 22.10.2019 15:00

Mathematics, 22.10.2019 15:00



Mathematics, 22.10.2019 15:00

English, 22.10.2019 15:00

Mathematics, 22.10.2019 15:00



Mathematics, 22.10.2019 15:00

Social Studies, 22.10.2019 15:00


Social Studies, 22.10.2019 15:00